Bibliography
what do scientists say about decompression?
They are gathered in short syntheses on the subject of decompression and the prevention of decompression sickness.
This knowledge has an informative value and does not pretend to be exhaustive.
1. Relationship between bubbles and Decompression sickness
Introduction
It is well known that the bubbles formed in the various parts of the body during a decompression can be pathogenic and may generate several forms of DCS. Circulating bubbles are probably just a part of the overall bubble production by the body but reflect a physiological stress. They play a role in numerous DCS mechanisms and are linked to various biochemical phenomena associated to DCS occurrence (blood-bubble interactions) and to endothelium impairment [1]. An overload of the pulmonary filter by bubbles cannot be associated to a safe decompression. For all these reasons, bubble detection has developed to assess decompression procedure adequacy [2].
Literature overview
Even if there was initially no clear evidence of a causal relationship between the amount of bubbles circulating in the blood stream and the occurrence of DCS, numerous Doppler and ultrasonic imaging studies support the association between venous gas emboli (VGE) levels and DCS risk [3] to [15]. The relative performance of Doppler and ultrasonic imaging was assessed through a dedicated study that didn’t point out a significant difference between these methods for bubble detections performed at rest [16].
The inter-individual variability regarding detectable bubble production is large [17]. Additionally, it seems that divers with an history of DCS appear more prone to produce detectable bubbles than divers without an history of DCS [18]. However, high levels of bubbles can be sustained without DCS symptoms [19][20].
Indeed, it is now largely accepted that VGE is a poor predictor of DCS (low specificity), but the absence of VGE is a good indicator of decompression safety (high sensitivity) [21][22]. This is why the amount of VGE detected is believed to be a useful decompression stress indicator for comparing decompression procedures or controlling their efficiency [2][23][24]. For example, the Defence and Civil Institute of Environmental Medicine (DCIEM, now DRDC – Toronto Research Centre) has used the Doppler ultrasound method to detect VGE in order to develop various decompression tables for the Royal Canadian Navy [25] to [28]. The potential of bubble detection to assess the relevance of decompression procedures has been pointed out using modern statistical approaches [22] [29] [30]. This offers interesting possibilities and makes feasible – in terms of cost, time, statistical relevance and health impairment control – the validation of decompression profiles to reach a given DCS risk target.
Discussion
While both statistical tools and bubble detection have proven to be useful, they remain characterized by limitations: the probabilistic approach is an a priori method that does not consider inter/intra individual variability with respect to DCS susceptibility while the bubble detection approach is an a posteriori method that does not consider pressure profile/decompression profile to assess DCS risk. However, it is well known that both VGE formation and DCS occurrence depend primarily upon the dive exposure (depth, duration, gas breathed), the decompression procedure (ascent rate, decompression stops, oxygen during stops) and physical characteristics of the diver (age, body mass index BMI). An in-depth analysis of a large dataset using a logistic regression method showed that the association between large VGE loads and the increase in probability of DCS persists after taking into account the dive parameters, such as the depth, the bottom time and the decompression time, and the individual covariates such as age and BMI [31]. The limitations of approaches linking directly bubble grades with DCS risk were pointed elsewhere [32]. In this last study, the nature of the experimental profiles was presented as playing a role in the DCS occurrence probability.
Ultrasonic detection of VGE in the precordial (PRE) region is commonly used in evaluation of decompression stress. While subclavian (SC) VGE detection can also be used to augment and improve the evaluation, a recent study compared VGE grades from both sites as decompression stress indicators. It was proved that the association of bubble grades with DCS occurrence is stronger for SC than PRE when exposure severity is taken into account [33]. The usefulness of SC VGE in decompression stress evaluation has so been underestimated in the past.
Conclusion
Bubble detection cannot be used to prevent or predict in real time DCS occurrence as the bubble specificity regarding DCS risk is low. However, it has been statistically proved that for a same exposure severity* (*ratio between depth, duration and total ascent time), the risk ratio between a high bubble grade detected and a low bubble grade detected is significant [33].
So, it is highly likely that a diver producing large quantities of bubbles on a routine basis ultimately increases his risk of DCS significantly compared to a routine low-level bubble production.
Bubble detection is so an interesting tool to help the diver by directing him towards a safer practice, by selecting alternative choices that may decrease at best his bubble production (additional stops, use of more oxygen during stops, nitrox, use of helium, GF settling).
References
- Møllerløkken A, Gaustad SE, Havnes MB, Gutvik CR, Hjelde A, Wisloff U, Brubakk AO. Venous gas embolism as a predictive tool for improving CNS decompression safety. European Journal of Applied Physiology. 2012; 112:401–409. – FULL TEXT
- Nishi RY, Brubakk AO, Eftedal OS. Bubble detection. In: Brubakk AO, Neuman TS, editors. Bennett and Elliott’s physiology and medicine of diving, 5th ed. London: WB Saunders; 2003. p. 501–29.
- Spencer MP, Johanson DC. Investigation of new principles for human decompression schedules using the Doppler ultrasonic blood bubble detector. Tech Report to ONR on Contract N00014-73-C-0094. Seatlle, WA: Institute for Environmental Medicine and Physiology; 1974. – FULL TEXT
- Nashimoto I, Gototh Y. Ultrasonic Doppler detection of blood bubbles in caisson work. In: Pearson R, editor. Early diagnosis of decompressions. Proceedings of the Twelfth Undersea Medical Society Workhsop. UMS 7-30-77. Bethesda MD: Undersea Medical Society; 1977. p. 171–83.
- Nashimoto I, Gotoh Y. Relationship between precordial Doppler ultrasound records and decompression sickness. In: Shilling CW, Beckett MW, editors. Underwater physiology VI: Proceedings of the Sixth Symposium on Underwater Physiology. Bethesda, Maryland: Federation of American Societies for Experimental Biology; 1978. p. 497–501.
- Powell MR, Johanson DC. Ultrasound monitoring and decompression sickness. In: Shilling CW, Beckett MW, editors. Underwater physiology VI: Proceedings of the Sixth Symposium on Underwater Physiology. Bethesda, Maryland: Federation of American Societies for Experimental Biology; 1978. p. 503–10.
- Gardette B. Correlation between decompression sickness and circulating bubbles in 232 divers. Undersea Biomedical Research. 1979; 6:99–107. – ABSTRACT
- Vann RD, Dick AP, Barry PD. Doppler bubble measurements and decompression sickness. Undersea Biomed Res. 1982; 9(Suppl 1): S24. – ABSTRACT
- Eatock BC. Correspondence between intravascular bubbles and symptoms of decompression sickness. Undersea Biomedical Research. 1984; 11:326-9.
- Masurel G. Contribution à l’étude du rôle physiopathologique des bulles générées chez l’animal et chez l’homme par un séjour en atmosphère hyperbare [PhD Thesis]. Lyon, Claude Bernard-Lyon I University; 1987. French.
- Sawatzky KD. The relationship between intravascular Doppler-detected gas bubbles and decompression sickness after bounce diving in humans. M.Sc. Thesis, York University, Toronto; 1991.
- Sawatzky KD. Nishi RD. Intravascular Doppler-detected bubbles and decompression sickness. Undersea and Hyperbaric Medical Society, Inc. Joint Annual Scientific Meeting with the International Congress for Hyperbaric Medicine and the European Undersea Biomedical Society held 11-18 August 1990. Okura Hotel, Amsterdam, The Netherlands. – ABSTRACT
- Conkin J, Powell MR, Foster PP, Waligora JM. Information about venous gas emboli improves prediction of hypobaric decompression sickness. Aviation, Space and Environmental Medicine. 1998; 69:8–16. – ABSTRACT
- Pilmanis AA, Kannan N, Krause KM, Webb JT. Relating venous gas emboli (VGE) scores to altitude decompression sickness (DCS) symptoms. [Abstract]. Aviation, Space and Environmental Medicine. 1999; 70:364.
- Eftedal OS, Lydersen S, Brubakk AO. The relationship between venous gas bubbles and adverse effects of decompression after air dives. Undersea and Hyperbaric Medicine. 2007; 34:99–105. – FULL TEXT
- Brubakk AO, Eftedal O. Comparison of three different ultrasonic methods for quantification of intravascular gas bubbles. Undersea and Hyperbaric Medicine. 2001; 28(3):131–136. – FULL TEXT
- Papadopoulou V, Germonpre P, Cosgrove D, Eckersley RJ, Dayton PA, Obeid G, Boutros A, Tang MX, Theunissen S, Balestra C. Variability in circulating gas emboli after a same scuba diving exposure. European Journal of Applied Physiology. 2018; 118(6):1255–1264. – ABSTRACT
- Gawthrope IC, Summers M, Macey DJ, Playford DA. An observation of venous gas emboli in divers and susceptibility to decompression sickness. Diving and Hyperbaric Medicine. 2015; 45(1):25–29. – FULL TEXT
- Bakovic D, Glavas D, Palada I, Breskovic D, Fabijanic D, Obad A, Valic Z, Brubakk AO, Dujic Z. High-grades bubbles in left and right heart in an asymptomatic diver at rest after surfacing. Aviation, Space and Environmental Medicine. 2008; 79:626-628. – ABSTRACT
- Ljubkovic M, Dujic Z, Møllerløkken A, Bakovic D, Obad A, Breskovic D, Brubakk AO. Venous and arterial bubbles at rest after no-decompression air dives. Medicine & Science in Sports & Exercise. 2011; 43(6):990-995. – ABSTRACT
- Pollock NW. Use of ultrasound in decompression research. Diving and Hyperbaric Medicine. 2007; 37:68–72. – FULL TEXT
- Blogg SL, Møllerløkken A. The use of venous gas emboli to validate dive computers. Proceedings of Validation of Dive Computers Workshop; Blogg SL, Lang MA, Møllerløkken A, editors. European Underwater and Baromedical Society; 2011. p. 93–7. – FULL TEXT
- Jones AD, Miller BG, Colvin AP. Evaluation of Doppler monitoring for the control of hyperbaric exposure in tunneling. Research Report RR598. UK Health and Safety Executive; 2007. – FULL TEXT
- Cooper PD, Van den Broek C, Smart DR, Nishi RY, Eastman D. Hyperbaric chamber attendant safety I: Doppler analysis of decompression stress in multiplace chamber attendants. Diving and Hyperbaric Medicine. 2009; 39:63–70. – FULL TEXT
- Lauckner GR, Nishi RY, Eatock BC. Evaluation of the DCIEM 1983 decompression model for compressed air diving (series A-F). DCIEM Report n 84-R-72. Downsview, Ontario, Canada: Defence and Civil Institute of Environmental Medicine; 1984. – FULL TEXT
- Lauckner GR, Nishi RY, Eatock BC. Evaluation of the DCIEM 1983 decompression model for compressaed air diving (series G-K). DCIEM Report n 84-R-73. Downsview, Ontario, Canada: Defence and Civil Institute of Environmental Medicine; 1984. – FULL TEXT
- Lauckner GR, Nishi RY, Eatock BC. Evaluation of the DCIEM 1983 decompression model for compressed air diving (series L-Q). DCIEM Report n° 85-R-18. Downsview, Ontario, Canada: Defence and Civil Institute of Environmental Medicine; 1985. – FULL TEXT
- Nishi RY, Eatock BC. The role of ultrasonic bubble detection in table validation. In: Schreiner HR, Hamilton RW, editors. Validation of decompression tables. Proceedings of the 37th Undersea and Hyperbaric Medical Society Workshop, UHMS Publication 74(VAL)1-1-88. Bethesda, MA: Undersea and Hyperbaric Medical Society; 1989. p. 133–7. – FULL TEXT
- Doolette DJ, Gault KA, Gutvik CR. Sample size requirement for comparison of decompression outcomes using ultrasonically detected venous gas emboli (VGE): power calculations using Monte Carlo resampling from real data. Diving and Hyperbaric Medicine. 2014; 44(1): 14–19. – FULL TEXT
- Eftedal OS, Tjelmeland H, Brubakk AO. Validation of decompression procedures based on detection of venous gas bubbles: a Bayesian approach. Aviation, Space and Environmental Medicine. 2007;78:94–9. – ABSTRACT
- Shannon JS. The relationship of inert gas and venous gas emboli to decompression sickness [PhD Thesis]. Durham, NC: Duke University; 2003. – FULL TEXT
- Doolette DJ. Venous gas emboli detected by two-dimensional echocardiography are an imperfect surrogate endpoint for decompression sickness. Diving and Hyperbaric Medicine. 2016; 46(1): 4–10. – FULL TEXT
- Hugon J, Metelkina A, Barbaud A, Nishi R, Bouak F, Blatteau J-E, Gempp E. Reliability of venous gas embolism in the subclavian area for decompression stress assessment following scuba diving. Diving and Hyperbaric Medicine. 2018; 48(3): 132–140. – FULL TEXT
2. Individual characteristics, bubble formations and decompression sickness risk
Introduction
Decompression sickness (DCS) is a major risk for health and safety of a scuba diver. It is caused by the formation and growth of microbubbles of inert gas in the diver’s tissues and blood during and after a decompression. The exposure of the diver’s body to hyperbaric condition, compressed gas breathing and variations of the ambient pressure is at the origin of DCS. The consequences of DCS range from skin itching to serious neurological damages. For recreational divers, the reported incidence of DCS is 3-4 cases per 10 000 dives, among which about 50% are of neurological type [1]. The exact pathological mechanisms are not completely understood: the risk depends on the exposure (dive profile, breathing gases, environmental conditions), but the diver’s susceptibility to DCS might be influenced by individual physiological characteristics and conditions. This is reflected by a significant inter-individual and intra-individual variability regarding circulating bubbles production on almost identical exposures [2].
The Divers Alert Network (DAN) accumulates a large database (DAN DB) of recreational dives with dive and diver-related information. Recently, a large-scale epidemiological study [3] investigated the factors influencing the risk of DCS and post-dive bubbles using the data from DAN DB. The study included 39 000 open circuit air/nitrox dives, from which 970 dives were accompanied with post-dive venous bubble measurements and 320 generated DCS cases. The results confirmed the findings of small controlled studies and of a previous epidemiological study of DCS risk factors analyzing 458 000 dives of English divers [4]. The investigated factors we have reviewed hereafter are important for a correct DCS risk management and should be taken into account in dive planning, for instance by selecting a suitable level of conservatism for dive procedures (at individual or at group level).
Literature overview
Both the quantity of dive-generated bubbles and the risk of DSC are increasing with age [3] [5] [6] [7], as confirmed by a comparative analysis taking into account the exposure severity [8]. The effect of aging on the pathophysiology of decompression is complex and poorly understood: it can be related to the development of health problems, to physiological changes and loss of fitness.
The gender-related difference in bubble production is less clear. A recent study didn’t find a significant difference [3] (but they ignored interactions with other factors). However, some studies showed that women produce less bubbles than men in younger ages but not in older, possibly because of menopause-related physiological changes [5] [6]. A DCS incidence higher in women than in men was reported [3] [4], but not in all studies [9]. The explanation of this contradiction could be complex: not only physiology, but diving habits and risk management may differ between women and men. For example, fewer women are making additional stops at the same level of experience [4]. When the diving experience is taken into account, women are at lower risk than men [4]. Moreover, there is some evidence that men tend to underreport mild DCS symptoms, introducing a bias into the analysis of incidence [4]. Concerning women specific risk factors, the effect of menstrual cycle on susceptibility of DCS is controversial and lacks of verified data [5] [10].
The Bodymass Index (BMI) and the percentage of fat are commonly used to measure adiposity, which can increase the total inert gas content in the diver’s body because of the high solubility of nitrogen in fat tissues. The divers with a higher BMI and percentage of fat tend to produce more bubbles and to have higher incidence of DCS [3] [6], even when the comparison is adjusted to exposure severity [8]. However, adiposity is strongly correlated with older age and lower physical fitness and their respective effects on decompression-related bubbles and susceptibility to DCS are difficult to isolate.
Physically fit divers are more resistant to decompression sickness: in particular a greater aerobic capacity (measured by maximum oxygen uptake) seems to be associated with a lower bubbles production [11]. The divers are advised to exercise regularly for better oxygen usage, inert gas elimination and to reduce the risk of exhaustion.
In presence of circulating venous bubbles in the diver’s body after decompression, the risk of neurological DCS is amplified when bubbles can penetrate into the arterial circulation (bubbles arterialization), reaching central organs such as the brain. Unfortunately, a diver could have a physiological predisposition to arterio-venous shunting: a small hole in the wall separating left and right ventricles in the heart (patent foramen ovale, PFO) or a communication between arteries and veins in the lungs (pulmonary shunts) [12] [13]. As PFO’s role in DCS exposure was debated in the literature for long time [14], we dedicated a special review note to this subject.
A controlled study on 400 divers showed that dehydration significantly increases the risk for developing DCS [15]. A small study on 9 divers suggested that a rehydration 90 min before diving reduces the bubbles production and significantly increases the plasma volume [16]. Divers should drink enough 1.5-2 hours before immersion to be correctly hydrated.
No epidemiological study answered conclusively whether the alcohol consumption affects diver’s susceptibility to DCS, but it is known that a pre-dive alcohol consumption increases the dehydration, leading to an increased risk of DCS. Alcohol consumption just after dive is provocative for bubble formation [17]. As a consequence, the divers are advised to avoid any alcohol for at least 12 hours before dive and 2 or more hours after dive.
Concerning smoking, smokers tend to present more severe symptoms that non-smokers [18].
Conclusion
Decompression sickness accident is a complex phenomenon. Many factors influence its occurrence, some of them being under the diver’s control, the others not. Many of such factors are related to both the increase of after-dive circulating bubbles and the increased risk of DCS, what consolidates the relationship established between circulating bubbles and DCS, for which we dedicated a special review note.
Divers should be aware of both kinds of factors and take them into account while planning dives, to avoid unnecessary risky exposure.
In short, the literature tends to propose the following recommendations:
- Keep your physical fitness with regular exercise;
- Hydrate yourself and avoid alcohol before dive;
- Avoid smoking;
- Adapt more conservative decompression when you get older;
- Dive less deep, less long and be more conservative if you don’t feel in your best shape, .
References
Buzzacott P., Denoble P.J. (editors). DAN Annual Diving Report 2018 Edition – A report on 2016 diving fatalities, injuries, and incidents, Durham, NC: Divers Alert Network, 2018; pp. 1-112 – FULL TEXT
Papadopoulou V., Germonpré P.,Cosgrove D., Eckersley R.J.,Dayton P.A., Obeid G., Boutros A.,Tang M.-X., Theunissen S., Balestra C., Variability in circulating gas emboli after a same scuba diving exposure, European Journal of Applied Physiology, 2018, vol. 118 (6): pp. 1255–1264. – ABSTRACT
Cialoni D., Pieri M., Balestra C., Marroni A., Dive Risk Factors, Gas Bubble Formation, and Decompression Illness in Recreational SCUBA Diving: Analysis of DAN Europe DSL Data Base, Frotiers in Physiology, 2017, vol. 8, article 1587. – FULL TEXT
Leger Dowse M.St., Bryson A., Gunby A., Fife W., Comparative Data from 2250 Male and Female Sports Divers: Diving Patterns and Decompression Sickness, Aviation, Space and Environmetal Medicine, 2002, vol. 73(8), pp. 743-749. – FULL TEXT
Dunford R.G., Vann R.D., Gerth W.A., Pieper C.F., Huggins K., Wacholtz C., Bennett P.B. The incidence of venous gas emboli in recreational diving, Undersea and Hyperbaric Medicine, 2002, vol. 29(4):pp. 247-59. – FULL TEXT
Boussuges A., Retali G., Bodéré‐Melin M., Gardette B., Carturan D., Gender differences in circulating bubble production after SCUBA diving, Clinical Physiology and Functional Imaging, 2009, vol.29(6): pp. 400-405. – ABSTRACT
Souday V., Koning N.J., Perez B., Grelon F., Mercat A., Boer C., Seegers V., Radermacher P., Asfar P., Enriched Air Nitrox Breathing Reduces Venous Gas Bubbles after Simulated SCUBA Diving: A Double-Blind Cross-Over Randomized Trial, Plos One, 2016, vol. 11(5):e0154761. DOI: 10.1371/journal.pone.0154761. – FULL TEXT
Shannon J.S., The relationship between inert gas and venous gas emboli to decompression sickness, PhD Thesis, Durham, NC, Duke University, 2003. –FULL TEXT
Mirasoglu B, Aktas S. Turkish recreational divers: a comparative study of their demographics, diving habits, health and attitudes towards safety, Diving and Hyperbaric Medicine, 2017, vol.47(3), pp. 173–179. – FULL TEXT
Lee V., St. Leger Dowse M., Edge C., Gunby A., Bryson P. Decompression sickness in women: a possible relationship with the men strual cycle, Aviation, Space and Environmental Medicine, 2003, vol. 74, pp. 1177-82. – ABSTRACT
- Caraturan D., Boussuges A., Vanuxem P., Barhen A., Burnet H., Gardette B., Ascent rate, age, maximal oxygen uptake, adiposity, and circulating venous bubbles after diving, Journal of Applied Physiology, 2002, vol. 93(4), pp. 1349-56. – FULL TEXT
Pollock N.W., Aerobic fitness and underwater diving, Diving and Hyperbaric Medicine, 2007, vol. 37 (3), pp. 117-124. – FULL TEXT
Germonpré P., Dendale P., Unger P., Balestra C. Patent foramen ovale and decompression sickness in sports divers Journal of Applied Physiology, 1998, vol. 84(5): pp. 1622-1626. – FULL TEXT
Madden D., Lozo M., Dujic Z., Ljubkovic M. , Exercise after SCUBA diving increases the incidence of arterial gas embolism, Journal of Applied Physiology, 2013, vol 115(5): pp. 712-722. – FULL TEXT
Denoble P.J., Holm J.R. eds., Patent Foramen Ovale and Fitness to Dive Consensus Workshop Proceedings, Durham, NC, Divers Alert Network, 2015, 160 pp. – FULL TEXT
Gempp E, Blatteau J, Pontier J ., Balestra C., Louge P., Preventive effect of pre-dive hydration on bubble formation in divers, British Journal of Sports and Medicine, 2009, vol. 43, pp. 224-228. – ABSTRACT
Sheldrake S., Pollock N. W, Alcohol and Diving, In: Steller D., Lobel L., eds. Diving for Science 2012, Proceedings of the American Academy of Underwater Sciences 31st Symposium, Dauphin Island, AL: AAUS. – FULL TEXT
Buch D.A., El Moalem H, Dovenbarger J.A., Uguccioni D.M., Moon R.E., Cigarette smoking and decompression illness severity: a retrospective study in recreational divers, Aviation Space and Environmental Medicine, 2003, vol. 74, pp. 1271–1274. – ABSTRACT
Specific reviews
Patent Foramen Ovale (PFO)
Introduction
The foramen ovale is a small hole located in the septum, the wall between the two upper chambers of the heart. Before birth, the lungs are not used to get blood rich in oxygen. Instead, this blood comes from the mother’s placenta and is delivered through the umbilical cord. The foramen ovale makes it possible for the blood to go directly from the veins to the right atrium of the fetus’ heart to the left atrium of the heart, bypassing the lungs. Normally, the foramen ovale closes as blood pressure rises in the left side of the heart after birth, or within a few years after birth. Once it is closed, the overall blood flows to the lungs without bypass, to get oxygen before it enters the left side of the heart and gets pumped to the rest of the body.
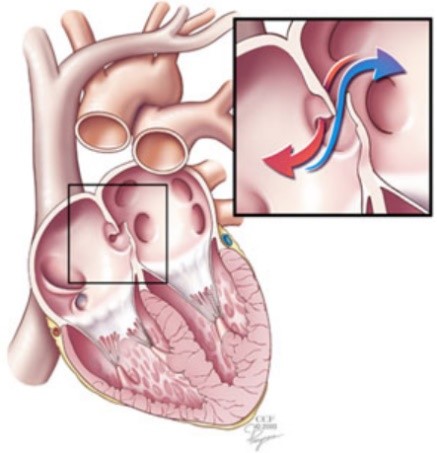
The foramen ovale remains open, or patent (patent foramen ovale or PFO), in about 25% of the adult population [1] (1). Most patients with a PFO do not have any symptoms. However, the condition may play a role in migraine headaches and it increases the risk of stroke, transient ischemic attack and heart attack. In divers, it has been associated with severe neurological decompression sickness, inner ear decompression sickness, and cutaneous symptoms [2]. Venous bubbles formed during and after decompression can pass through the foramen ovale shunt -so not filtered by the lung- and invade the peripheral arterial circulation. They can reach tissues supersaturated with inert gases and, as a result, there is an amplified risk of bubble emboli in these zones.
Literature overview
The correlation between the presence of a PFO and an increased risk of occurrence of a serious form of decompression sickness was mentioned more than thirty years ago [3], in particular concerning neurological forms which develop rapidly after the return to the surface [4].
The various forms of DCS for which a link has been demonstrated regarding the presence of PFO have been recently reviewed [2]. Cerebral DCS seem more linked to PFO presence than spinal cord DCS [5]. The correlation to the risk of spinal cord DCS is less marked but seems to exist [6] [7]. Regarding brain and inner ear injuries, where supersaturation levels can be locally high, mechanisms have been proposed and discussed [2] [8]. The relationship between cutaneous forms and PFO was initially more puzzling. A recent study leads to hypothesize a cerebrally mediated mechanism [9].
The DCS risk ratio for diver with PFO compared to diver without PFO is not precisely known but several studies tend to point a two 2.5 times risk increase at least [10] [11] and even more than five [12] [13]. Severe DCS forms are definitely more frequent when a PFO is present [14]. Additionally, the size of the PFO appears to play a major role regarding DCS risk ratio determination [12][15]. In a recent study, on the 200 divers who had an atrial defect closure (PFO and ASD) following shunt-related DCS, about 50% had an atrial defect 10mm or larger, whereas about 1% of the general population appear to have PFO diameter in this range (about 25% of the population have a PFO but with a diameter mainly between 2mm and 6mm) [15]. Nevertheless, serious DCS forms are sometimes associated with small size PFO [16].
PFO closure appears to be an efficient solution to strongly limit the passage of bubbles towards the arterial aspect [17] and to prevent major DCS and return to unrestrictive diving [16][18], while conservative diving profiles without PFO closure seems also to lead to satisfying level of safety regarding severe DCS forms [18]. It is also associated with a decrease of asymptomatic brain lesions [19]. PFO closure surgery is characterized by a low rate of procedural complication [20].
Discussion
DCS risk characterization linked to a PFO presence remains a major research topic. A rigorous statistical analysis is not yet available. A routine way of screening PFO (presence, dimension, fully, partially, intermittently opened…), with or without a DCS case history, with or without invasive means, is not feasible or even advisable.
Indeed, whereas the scientific community agrees on the fact that PFO is a DCS risk factor, a routine screening of diver is not preconized [20]. Only divers with an history of severe DCS form are eligible for a routine screening, through bubble contrast transthoracic echocardiography with provocative manoeuvres. In case of positive PFO result, PFO close using transcatheter is recommended for return to normal diving [21].
However, a conservative approach of diving, with less severe diving profiles leading to gas load limitation, appears to be an interesting alternative to limit DCS risk [22].
Finally, it must be noticed that other right-to-left shunt pathways may exist in the body, in particular at pulmonary level [23][24], the impact of oxygen and the level of effort on this type of shunt having been studied [24] [25] [26]. Currently, the DCS risk associated with this anatomical reality (with a dispersion of its importance among the population) and its role in microbubble arterialization is poorly known.
References
- Homa S, Messé SR, Rundek T, Sun YP, Franke J, Davidson K, Sievert H, Sacco RL, Di Tullio MR. Patent foramen ovale. Nat Rev Dis Primers. 2016; 2: 15086. – ABSTRACT
- Wilmshurst PT. The role of persistent foramen ovale and other shunts in decompression illness. Diving Hyperb Med. 2015; 45(2):98-104. – FULL TEXT
- Moon RE, Camporesi EM, Kisslo JA. Patent foramen ovale and decompression sickness in divers. Lancet. 1989 Mar 11, 1(8637):513-514. – ABSTRACT
- Wilmshurst PT, Byrne JC, Webb-Peploe MM. Relation between interatrial shunts and decompression sickness in divers. Lancet. 1989 Dec 2, 2(8675):1302-1306. – ABSTRACT
- Germonpré P, Dendale P, Unger P, Balestra C. Patent foramen ovale and decompression sickness in sports divers. Journal of Applied Physiology. 84(5): 1622-1626. – FULL TEXT
- Wilmshurst PT, Bryson P. Relationship between the clinical features of neurological decompression illness and its causes. Clinical Science. 2000; 99:67-75. – FULL TEXT
- Wilmshurst PT. Clinical experience of right-to-left shunts in divers with decompression illness. Patent Foramen Ovale and Fitness to Dive Consensus Workshop, Montreal June 17 2015. pp. 21-33. – FULL TEXT
- Mitchell SJ, Doolette DJ. Pathophysiology of inner ear decompression sickness: potential role of the persistent foramen ovale. Diving Hyperb Med. 2015; 45(2): 105-110. – FULL TEXT
- Kemper TCPM, Rienks R, Van Ooij PJAM, Van Hulst RA. Cutis marmarota in decompression illness may cerebrally mediated: a novel hypothesis on the aetiogoloy of cutis marmarota. Diving Hyperb Med. 2015; 45(2):84-88. – FULL TEXT
- Bove AA. Risk of decompression sickness with patent foramen ovale. Undersea and Hyperbaric Medicine. 1998; 25(3): 175-178. – ABSTRACT
- Germonpre P. Incidence of DCS in divers with RLS – A prospective study. Patent Foramen Ovale and Fitness to Dive Consensus Workshop, Montreal, June 17 2015. pp. 47-58. – FULL TEXT
- Torti SR, Billinger M, Schwerzmann M, Vogel R, Zbinden R, Windecker S, Seiler C. Risk of decompression illness among 230 divers in relation to the presence and size of patent foramen ovale. Eur Heart J. 2004; 25: 1014-1020. – FULL TEXT
- Honek J, Sramek M, Sefc L, Januska J, Fiedler J, Horvath M, Tomak A, Novotny S, Honek T, Veselka J. High-grade patent foramen ovale is a risk factor of unprovoked decompression sickness in recreational divers. J Cardiol. 2019; 74(6): 519-523. –ABSTRACT
- Liou K, Wolfers D, Turner R, Bennett M, Allan R, Jepson N, Cranney G. Patent foramen ovale influences the presentation of decompression illness in SCUBA divers. Heart, Lung and Circ. 2015; 24: 26-31. – FULL TEXT
- Wilmshurst PT, Morrison WL, Walsh KP, Pearson MJ, Nightingale S. Comparison of the size of persistent foramen ovale and atrial septal defects in divers with shunt-related decompression illness and in the general population. Diving Hyperb Med. 2015; 45(2):89-93. – FULL TEXT
- Wilson C, Sayer MDJ. Cerebral arterial gas embolism in a professional diver with a persistent foramen ovale. Diving Hyperb Med. 2015; 45(2): 124-126. – FULL TEXT
- Honek J, Smarek M, Sefc L, Januska J, Fielder J, Horvath M, Tomek A, Novotny S, Honek T, Veselka J. Effect of catheter-based patent foramen ovale closure on the occurrence of arterial bubbles in scuba divers. Journal of The American College of Cardiology Intervention. 2014; 7(4): 403-408. – FULL TEXT
- Koopsen R, Stella PR, Thijs KM, Rienks R. Persistent foramen ovale closure in divers with a history of decompression sickness. Neth Heart J. 2018; 26: 535-539. – FULL TEXT
- Billinger M, Zbinden R, Mordasini R, Windecker S, Schwerzmann M, Meier B, Seiler C. Patent foramen ovale closure in recreational divers: effect on decompression illness and ischaemic brain lesions during long-term follow-up. 2011; 97(23): 1932-1937. – ABSTRACT
- Pearman A, Bugeja L, Nelson M, Szantho GV, Turner M. An audit of persistent foramen ovale closure in 105 divers. Diving Hyperb Med. 2015; 45(2): 94-97. – FULL TEXT
- Smart D, Mitchell S, Wilmshurst P, Turner M, Banham N. Joint position statement on persistent foramen ovale (PFO) and diving. South Pacific Underwater Medicine Society (SPUMS) and the United Kingdom Sports Diving Medical Committee. Diving Hyperb Med. 2015; 45(2): 129-131. – FULL TEXT
- Klingmann C, Rathmann N, Hausmann D, Bruckner T, Kern R. Lower risk of decompression sickness after recommendation of conservative decompression practices in divers with and without vascular right-to-left shunt. Diving Hyperb Med. 2012; 42(3): 146-150. – FULL TEXT
- Lovering AT, Stickland MK, Kelso AJ, Eldridge MW. Direct demonstration of 25 and 50µm arteriovenous pathways in healthy human and baboon lungs. Am J Physiol Heart Circ Physiol. 2007; 292: H1777-H1781. – FULL TEXT
- Lovering AT, Haverkamp HC, Romer LM, Hokanson JS, Eldridge MW. Transpulmonary passage of 99mTc macroaggregated albumin n healthy humans at rest and during maximal exercise. Journal of Applied Physiology. 2009; 106: 1986-1992. – FULL TEXT
- Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. Journal of Applied Physiology. 2008; 104: 1418-1425. – FULL TEXT
- Eldridge MW. Inducible intrapulmonary arteriovenous shunt pathways: are they important in DCS?. Patent Foramen Ovale and Fitness to Dive Consensus Workshop, Montreal, June 17 2015. pp. 65-72. – FULL TEXT
(1) In fact, there are two kinds of such holes in the heart. One is called an atrial septal defect (ASD), and the other is a patent foramen ovale (PFO). Although both are holes in the wall of tissue (septum) between the left and right upper chambers of the heart (atria), their causes are quite different. An ASD is a failure of the septal tissue to form between the atria, and as such it is considered a congenital heart defect, something that you are born with. Generally an ASD hole is larger than that of a PFO.
3. Exposure and environment, bubble formations and decompression sickness risk
Specific reviews
Deep stops
Introduction
Ten years ago, DAN published a text that summarized perfectly the deep stops issue [1]:
“Throughout history, the key concern for divers has been how to get back to the surface without suffering decompression sickness (DCS). The British scientist J.S. Haldane combined empirical data with scientific studies to develop step-by-step decompression procedures that, along with the accumulated experience and work of many scientists, led to the development of modern decompression tables and computer algorithms.
Decompression sickness still occurs in recreational divers, but at a rate of 1 to 4 per 10,000 dives; these cases of DCS are often mild and treatable. However, the possibility of severe injury — though rare — makes divers eager to hear about any measure that might further reduce the risk. One possibility is the deep stop.
In the mid-1990s, Richard Pyle, a biomarine scientist who frequently made dives to great depths in search of fish species, noticed that sometimes he felt fatigued after dives, and at other times he felt normal. An excellent observer and trained scientist, he figured out that on dives when he had to stop during his ascent to deflate the swim bladders of his specimens, he felt much better. Soon he introduced a brief stop halfway to the surface on all his dives and formed the strong opinion that this significantly reduced his post-dive fatigue. He shared his experience with fellow divers, and the practice of deep stops became widespread among technical divers before it could be scientifically tested.
What is a deep stop? In the minds of most who practice it, the deep stop is an additional stop during ascent, introduced by divers beyond what their computer algorithm demands. However, there are now computer algorithms that claim to include deep stops, though neither these algorithms nor the practice of deep stops has been thoroughly validated.
Discussion about deep stops is not new to the scientists studying decompression safety. Since Haldane first established decompression tables, the depth of the first stop has been debated. The answers varied over time, depending on prevailing contemporary dive practices and concerns. Haldane, for example, assumed that tissues may sustain a certain level of supersaturation or critical volume of surplus gas before bubbles occur. That is why his decompression model applied a relatively quick ascent to depths he believed would drive inert gas out of the body.
Later it became clear that bubbles probably occur much earlier than Haldane assumed, and these findings led to the creation of so-called bubble models. Many dive computers on the market incorporate deeper stops than do earlier Haldanian models. Some of them are based on bubble models, while others adjust parameters of non-bubble models to achieve similar effects. However, to mimic the deep-stop practices adopted by some technical divers, some computers add stops deeper than what their models call for or give divers this option.
So the big question before divers today is: How effective are deep stops at preventing DCS, whether called for by a bubble algorithm or when used by divers independent of what their computers suggest?”
This summary remains applicable nowadays. As will be explained hereafter, the controversy on deep stop relevance is a major debate, pointing that the decompression theory is yet a “living” topic. It is a frequently discussed question in the diving field [2], with currently less firm positions: a compromise has to be found between all the pro and cons theoretical views, reflected on a practical point of view by the gradient factors adjustment issue.
Literature overview
In the early 1960’s, more than twenty years before Pyle’s observations, Brian Hills analyzed the decompression procedures used by the Pearl fishermen in the Torres Strait and introduced a new concept of decompression modeling [3]. These divers practiced their first stop deeper than commonly done at that time, with, in addition, a higher pressure drop between the last stop and the surface than previously allowed. The zero supersaturation concept proposed by Hills assumed that bubbles can form even for very low supersaturation levels. As a consequence, the rate of the first part of the decompression has to be reduced significantly with a first stop rather deep. Even if Hills work never produced operational decompression tables, his approach inspired a generation of researchers who focused on bubble modeling and the necessity to limit the ambient pressure decrease amplitude during the ascent, in order to limit bubble generation and growth [4]. They revisited Haldane theory or at least tried to redefine the acceptable supersaturation thresholds. It was a new attractive and challenging topic.
In this context, David Yount, at the University of Hawaii, started fundamental work on decompression in the late 70’s. One of his objectives was the selection of criteria for microbubble formation, in the context of a supersaturation state. He assumed that the microbubbles forming in the body during a decompression are produced from preexisting micronuclei that are activated. Yount opened new perspectives by studying gelatin samples, compressed more or less rapidly, saturated with an inert gas and then decompressed. His experiments allowed, for the first time, the numbering of the micronuclei recruited and transformed into microbubbles under various supersaturation levels. A new decompression model called VPM (Varying Permeability Model) was finally proposed for man, using his theoretical foundations [5]. The VPM model, proposed by Eric Maiken and Erik Baker in the USA under version A then B, has been widely used by recreational technical SCUBA divers who dive rather deep with trimix and heliox mixtures: the TEK divers. It generates deep/short decompression stops compared to the more standard procedures as it limits the supersaturation levels to reduce the number of micronuclei recruited.
As a continuation of Yount’s approach and to marry several views, Bruce Wienke offered to the recreational SCUBA diving market a new algorithm RGBM widely used as a basis for several decompression computers from the 90’s.
Nevertheless, in parallel of bubble modelling motivations, Edward Thalmann works in USA for the US Navy and Albert Bühlmann works in Europe didn’t recuse Haldanian fundamentals. It is a matter of fact that they remain nowadays the basis of most of the current operational tables for both professional and recreational divers, and of most of the decompression computers.
Thalmann EL-RTA and the VVAL algorithms are of neo-Haldanian type, with exponential-linear gas exchange kinetics and various matrices of maximum permissible tissue tensions (M-value Workman approach). A slower tissue desaturation was introduced, with linear gas exchange, reflecting that bubble formation slows the inert gas removal kinetics. This constitutes a major evolution of neo-Haldanian models, pointing the fact that adaptations of the original approach are required.
In the recreational field, TEK divers, initially excited by the renewal proposed by VPM and RGBM deep stops, have finally selected Bühlmann ZH-L16 algorithm to a large extent, with the gradient factors (GF) option. They build their in-house decompression procedures with adjustment possibilities for the first stop depth (GF Low) and the total decompression time (GF High) [6]. The question of the decompression curve shape appears a central issue. This context has motivated several studies to investigate on the deep stops relevance.
In 2000’s, the French Navy tested four 50msw and 60msw air dive protocols on 12 divers with experimental ascent profiles (EAP) tested in the wet compartment of a hyperbaric chamber and with bubble monitoring to assess the benefits offered by deep stops [7][8]. The interest of deep stops was not demonstrated, with three of the EAPs showing no difference, one produced increase bubbling and one produced a joint pain DCS case compared to French Navy tables MN90.
It must be mentioned that the decompression profiles tested by the French Navy didn’t reflect the exact practice of technical divers (not fixed and clear at all) or the decompression protocol proposed by Richard Pyle [9]. This remark applies to the results observed by the US Navy through a dedicated study [10]. A 170fsw / 30min experimental exposure was tested according to VVAL18 Thalmann algorithm regarding the decompression on one side, and according to a probabilistic bubble model (BVM3) producing deep stops on the other side, with the same decompression duration. DCS incidence following these two schedules were compared. DCS incidence of the deep stops was significantly higher than that of the shallow stop conventional approach. It was deduced that the slower tissue gas kinetics is impacted by a deep stop, what is easily understandable if one follows a neo-Haldanian view (1). This US Navy experimental study follows a previous study tending to show that the introduction of a deep stop limits the production of bubbles on profiles 210fsw / 50min and 170fsw / 30min [11].
A more recent study tends once more to point no major advantages of a deep stop profile compared to a profile computed via Bühlmann algorithm adjusted with GF, for a 50msw/25min trimix dive: bubble production detected via 2D echocardiography was not significantly different and deep stops produced even more marked inflammatory responses [12].
Discussion
In 2008, the scientific community agreed to conclude: « In respect of decompression diving there is conflicting evidence regarding the relative efficiency of decompression regimens that include empirical or model-derived deep stops (as defined) and decompression regimens prescribed by gas content models” [13]. To date, this conclusion has still not been questioned.
It can be remarked that Pyle’s procedure, in agreement with theoretical views regarding microbubble production and growth dynamics, has never been really tested to prove its benefits compared to more conventional procedures.
As pointed by Erik Baker, there is an infinite possibility to produce deep stops even with a neo-Haldanian approach, using a Bühlmann algorithm modulated with a GF option [6]. The introduction of deep stops has to be compensated by a lengthening of the last stop close to the surface, probably to allow the egress of extra the gas load accumulated or contained in some tissues during the deep stop period.
The question of the deep stops interest becomes major if one focuses on the total decompression time required: for a same decompression duration, is a deep stop decompression profile better than conventional shallow stop? Rare studies tend to bring a response [10][14].
DAN interest regarding deep stop benefits was initially presented in 2000 [15]. The introduction of extra deep stops appeared advantageous for what concerns bubble production [16]. However, the lengthening of the decompression duration was significative in these studies. So, the question is the following: is it the deep stop by itself that limits the bubble production or is it the associated decompression duration increase, required according to most algorithms, that proves safer?
Additionally, if one believes that microbubble production can be minimized by opting for a deep stop approach, what is the optimal depth for the first stop, for a given diving profile?
It seems that currently, nobody can demonstrate if this optimal solution exists, as well as the total decompression duration advisable for this optimal way. Microbubbles form all the more profusely than the ambient pressure decrease is large but, in the same time, inert gas elimination is favorized by large ambient pressure decrease. So what is the best compromise? And how the inter and intra individual variabilities impact this optimal? Bubble detection -as a routine way of monitoring diver- should help to give a response to the question. A large database can be collected through the following of recreational diver activity and bubble production (large population, various diving profiles, various GF options), then analyzed.
References
- Denoble P. Deep stops. Alert Diver Online. Article 255; 2010. – FULL TEXT
- Powell M. Delving deeper into deep stops. Divernet. Reprinted from Diver; July 2018. – FULL TEXT
- Hills BA. A thermodynamic and kinetic approach to decompression sickness. Libraries Board of South Australia; 1966.
- Hugon J. Decompression models: review, relevance and validation capabilities. Undersea and Hyperbaric Medicine. 2014; 41(6): 531-556. – ABSTRACT
- Yount DE, Hoffman DC. On the use of a bubble formation model to calculate diving tables. Aviation Space Environmental Medicine. 1986; 57: 149-156. – FULL TEXT
- Baker EC. Clearing up the confusion about deep stops. – FULL TEXT
- Blatteau J-E,Hugon M, Gardette B, Sainty J-M, Galland F-M. Bubble incidence after staged decompression from 50 or 60msw: effet of adding deep stops. Aviation Space Environmental Medicine. 2005; 76(5): 490-492. – ABSTRACT
- Blatteau J-E,Hugon M, Gardette B. Deep stops during decompression from 50 to 100msw didn’t reduce bubble formation in man. Proceedings Decompression and the Deep Stop; Salt Lake City; 24-25 June 2008. – FULL TEXT
- Pyle R. The importance of deep safety stops: rethinking ascent patterns from decompression dives. SPUMS Journal. 1997; 27(2): 112-115. – FULL TEXT
- Doolette DJ, Gerth WA, Gault KA. Redistribution of decompression strop time from shallow to deep stops increases incidence of decompressions sickness in air decompression dives. Report NEDU TR-1106; Navy Experimental Diving Unit; 2011. – FULL TEXT
- Neuman T. Early observations on the effect of “deep” decompression upon Doppler ultrasonic bubble signals following 210/50 and 170/30 dives. Decompression and the Deep Stop, Salt Lake City; 24-25 June 2008; Proceedings; pp 5-14. – FULL TEXT
- Spisni E, Marabotti C, De Fazio L, Valerii MC, Cavazza E, Brambilla S, Hoxha K, L’Abbate A, Longobardi P. A comparative evaluation of two decompression procedures for technical diving using inflammatory responses: compartmental versus ratio deco. Diving and Hyperbaric Medicine. 2017; 47(1): 9-16. – FULL TEXT
- Consensus statements: statement regarding the efficacity of “deep stops” appropriate for the release to the diving community. Decompression and the Deep Stop, Salt Lake City; 24-25 June 2008; Proceedings; pp 324. – FULL TEXT
- Schellart NAM, Brandt Corstius J-J, Germonpré P, Sterk W. Bubble formation after a 20m dive: deep-stop vs shallow-stop decompression profiles. Aviation Space Environmental Medicine. 2008; 79: 488-494. – FULL TEXT
- Marroni A, Cali Carleo R, Balestra C, Longobardi P, Voellm E, Pieri M, Pepoli R. Effects of the variation of ascend speed and profile on the production of circulating venous gas emboli and the incidence of DCI in compressed air diving. Phase 1. Introduction of extra deep stops in the ascent profile without changing the original ascent rates. DSL Special Project 01/2000. European Underwater and Baromedical Society, EUBS Annual Scientific Meetings; Malta; 14-17 September 2000. – FULL TEXT
- Marroni A, Bennett PB, Cronje FJ, Cali Carleo R, Germonpre P, Pieri M, Bonuccelli C, Balestra C. A deep stop during decompression from 82fsw (25m) significantly reduces bubbles and fast tissue gas tensions. Undersea and Hyperbaric Medicine. 2004; 31(2): 233-243. – FULL TEXT
(1) GF approach coupled to a Bühlmann algorithm can counter-balance this drawback by lengthen the total decompression duration.
